What is Lithium Battery Technology?
Lithium batteries stand apart from other battery chemistries due to their high energy density and low cost per cycle. However, “lithium battery” is an ambiguous term. There are about six common chemistries of lithium batteries, all with their own unique advantages and disadvantages. For renewable energy applications, the predominant chemistry is Lithium Iron Phosphate (LiFePO4). This chemistry has excellent safety, with great thermal stability, high current ratings, long cycle life, and tolerance to abuse.
Outstanding lithium iron phosphate (LiFePO4) electrochemistry
Lithium Iron Phosphate (LiFePO4) is extremely stable lithium chemistry when compared to almost all other lithium chemistries. The battery is assembled with a naturally safe cathode material (iron phosphate). Compared to other lithium chemistries iron phosphate promotes a strong molecular bond, which withstands extreme charging conditions, prolongs cycle life, and maintains chemical integrity over many cycles.
This is what gives these batteries their great thermal stability, long cycle life, and tolerance to abuse. LiFePO4 batteries are not prone to overheat, nor are they disposed to ‘thermal runaway’ and therefore do not over-heat or ignite when subjected to rigorous mishandling or harsh environmental conditions.
The perfect replacement for lead-acid batteries
Unlike flooded lead-acid and other battery chemistries, lithium batteries do not vent dangerous gases such as hydrogen and oxygen. There’s also no danger of exposure to caustic electrolytes such as sulfuric acid or potassium hydroxide. In most cases, these batteries can be stored in confined areas without the risk of explosion and a properly designed system should not require active cooling or venting.
Security and flexibility
The LFP battery has a nominal voltage of 3.2V and can be connected in series to form a higher voltage to meet the needs of a variety of scenarios
These voltages are available for typical 12V, 24V and 48V inverters.
12V LiFePO4 Battery
To achieve a 12V battery, you typically need 4 cells in series and a nominal 12.8V battery.
36V LiFePO4 Battery
To achieve a 36V battery, you typically need 12 cells in series and a nominal battery of 38.4V.
24V LiFePO4 Battery
To achieve a 24V battery, you typically need 8 cells in series and a nominal battery of 25.6V.
48V LiFePO4 Battery
To achieve a 48V battery, you typically need 16 cells in series and a nominal battery of 51.2V.
From lead-acid batteries to lithium batteries
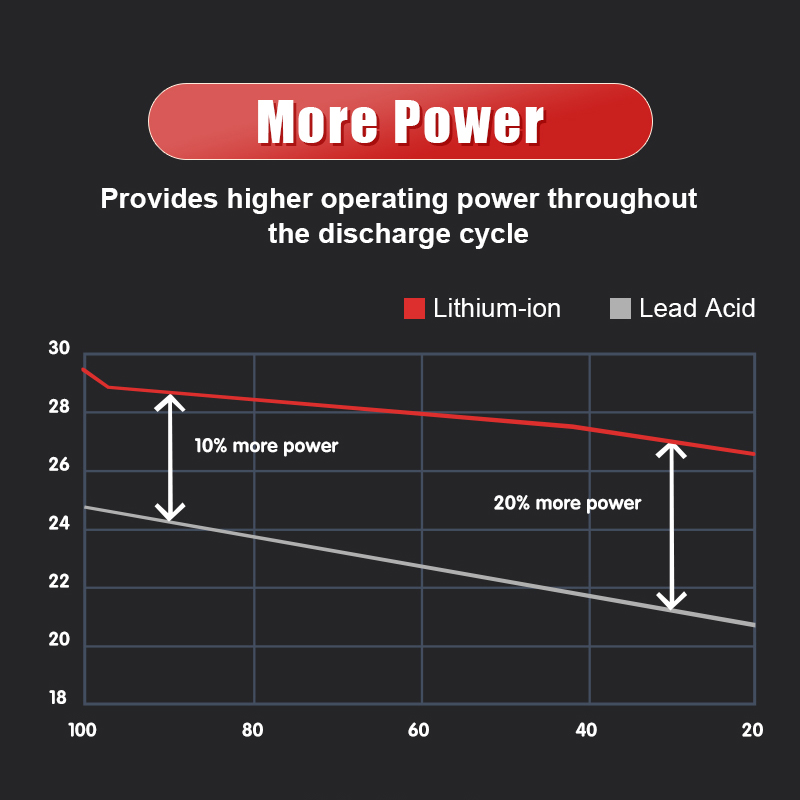
Lithium batteries are often used as direct replacements for lead-acid batteries because they have very similar charging voltages. Four-cell LiFePO4 batteries (12.8V), typically have a maximum charge voltage between 14.4-14.6V (depending on the manufacturer’s recommendations).
- Maintains higher voltage than lead acid under load
- The battery reaches the maximum voltage and does not need further charging
- Compared with lead-acid batteries, there is no problem of under-cycling
- LiFePO4 batteries do not require periodic recharging
- Lithium iron phosphate batteries have a round-trip energy efficiency of 95-98%
- Charging time from fully discharged to fully charged can be as short as two hours
- LiFePO4 batteries can be almost completely discharged at their rated value

The "brain" of Li-ion battery - BMS
The safety and reliability of lithium batteries are a big concern, thus all assemblies should have an integrated Battery Management System (BMS). The BMS is a system that monitors, evaluates, balances, and protects cells from operating outside the “Safe Operating Area”. The BMS is an essential safety component of a lithium battery system, monitoring and protecting the cells within the battery against over current, under/over voltage, under/over temperature and more. A LiFePO4 cell will be permanently damaged if the voltage of the cell ever falls to less than 2.5V, it will also be permanently damaged if the voltage of the cell increases to more than 4.2V. The BMS monitors each cell and will prevent damage to the cells in the case of under/overvoltage.
Balance the battery pack during charging to avoid overcharging.
The cells of a LiFePO4 battery will not automatically balance at the end of the charge cycle. There are slight variations in the impedance through the cells and thus no cell is 100% identical. Therefore, when cycled, some cells will be fully charged or discharged earlier than others. The variance between cells will increase significantly over time if the cells are not balanced.

In lead-acid batteries, current will continue to flow even when one or more of the cells are fully charged. This is a result of the electrolysis taking place within the battery, the water splitting into hydrogen and oxygen. This current helps to fully charge other cells, thus naturally balancing the charge on all cells. However, a fully charged lithium cell will have very high resistance and very little current will flow. The lagging cells will therefore not be fully charged. During balancing the BMS will apply a small load to the fully charged cells, preventing it from overcharging and allowing the other cells to catch up.
Join Us
Lithium batteries offer many benefits
over other battery chemistries.
They are a safe and reliable battery solution, with no fear of thermal runaway and/or catastrophic meltdown, which is a significant possibility from other lithium battery types. These batteries offer extremely long cycle life, with some manufacturers even warranting batteries for up to 10,000 cycles. With high discharge and recharge rates upwards of C/2 continuous and a round-trip efficiency of up to 98%, it’s no wonder these batteries are gaining traction within the industry. Lithium Iron Phosphate (LiFePO4) is a perfect energy storage solution.
